Technology Platform
Systasy’s technology is unique as it uses millions of molecular barcodes combined with human disease models to analyze hundreds of pathological processes simultaneously. It connects thousands of compound activities to an individual patient’s clinical and genomic data. Systasy’s technology lowers failure in subsequent clinical phases and leads to cost savings for our partners.
Systasy’s capabilities in generating powerful cellular models and in pathway-based drug discovery are based on proprietary technologies and databases.
Technology Assets
- Pathway-based drug discovery engine and viral tropism screening powered by leading DNA-barcoding technology.
Technology Assets
Pathway-based drug discovery engine and viral tropism screening powered by leading DNA-barcoding technology.
Patient Assets
- Deeply characterized real world data and tissue material from patient cohort with mental disorders for iPSC-based human disease models.
Patient Assets
Deeply characterized real world data and tissue material from patient cohort with mental disorders for iPSC-based human disease models.
Know-how Assets
- In depth knowledge in highly automated Single-cell sequencing .
- AI-based algorithms for biomarker identification, patient stratification, and the screening and analysis of novel compounds and Drug Repurposing
Know-how Assets
AI-based algorithms for biomarker identification, patient stratification, and the screening and analysis of novel compounds and Drug Repurposing.
Barcoding Technology
We utilize our novel barcoding technology to map signaling networks in disease models derived from our own extensive patient database or provided by our clients and partners. By identifying the dysregulated pathways involved in diseases, we aim to enhance our understanding of the underlying mechanisms and enable the development of effective treatments.
Systasy massively reduces experimental numbers by high-content multiplex screening. We are using validated barcoded pathway sensors that allow for tracking of multiple features in parallel, including signaling pathway activities, upstream targets, cellular identities and experimental conditions.
Barcode-coupled biosensors can be introduced into any cell type to identify and target multiple disease mechanisms in human cell networks. The same principles are applied to screen compound libraries and optimize lead compounds to bring dysregulated pathways back in sync.
We can address more than 100 individual signaling events, that are grouped into seven major signaling clusters covering regulation of cellular stress, cell fate, metabolism, immune response, stem cell pluripotency, synaptic activity & calcium signaling, and immediate early gene (IEG) response.
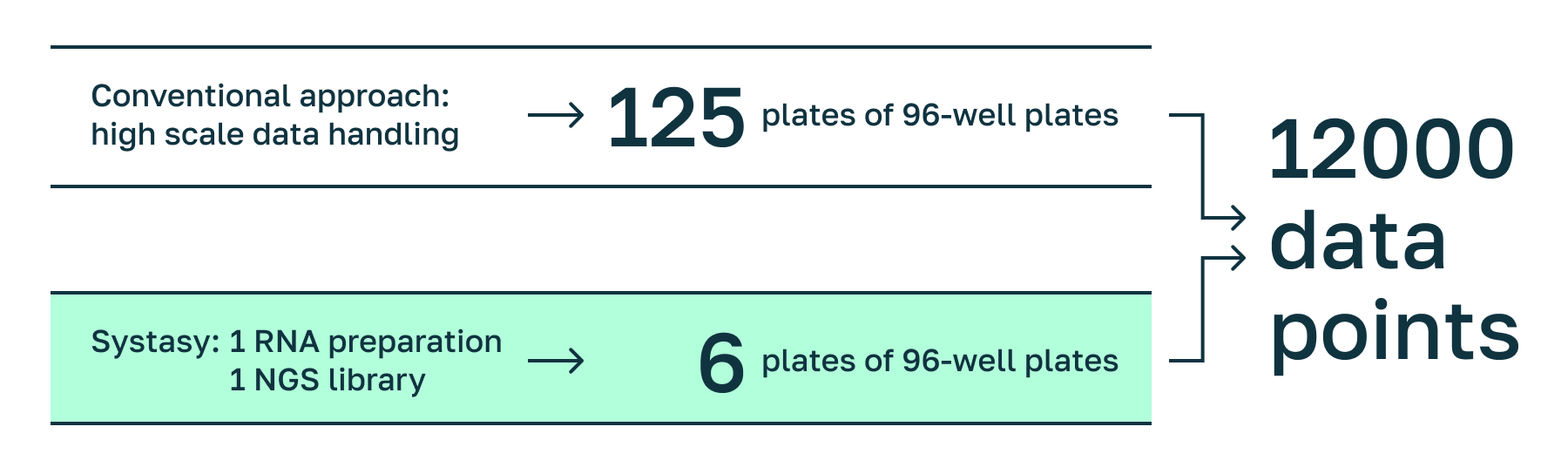
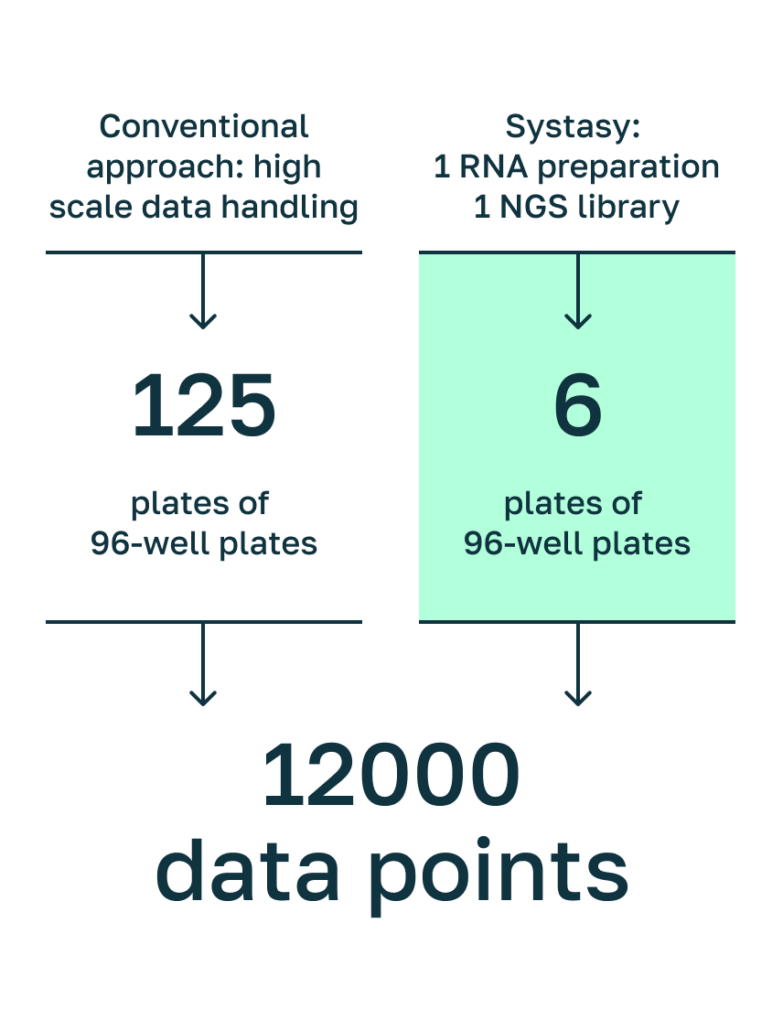
Reducing handling & data depth by Systasy‘s pathway-focus and barcoding approach.
Reducing handling & data depth by Systasy‘s pathway-focus and barcoding approach.
Why is our approach unique?
Why is our
approach unique?
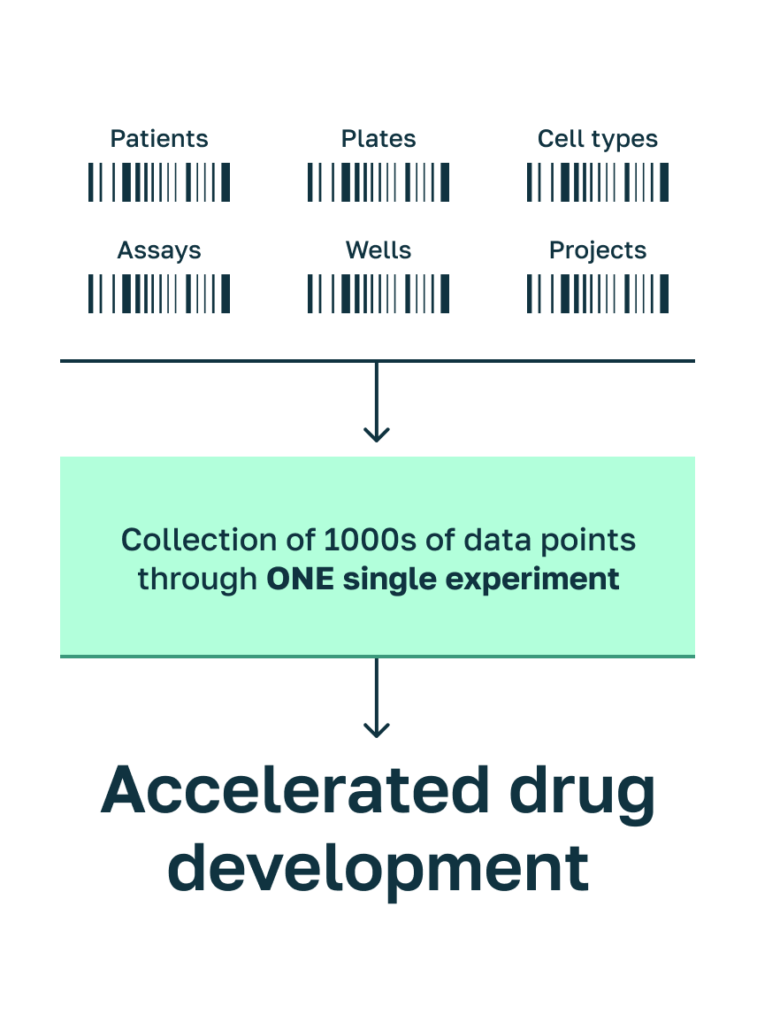
The unmatched depth of our technology enables to decode several parameters within one experiment.
The unmatched depth of our technology enables to decode several parameters within one experiment.
Defined treatment conditions such as different concentrations of a compound and time points are measured within one experiment using our proprietary Systasy Barcoded-based multiplex platform.
The highest-performing sensors can subsequently be used for simple, robust and cost-effective screening of compound effects in human disease models. Further, drug effects on specific patient groups can be predetermined in our human disease models to enable patient stratification according to their response profiles, ultimately increasing the likelihood of clinical success by meeting patient-specific demands.
Human Disease Models
Systasy employs barcode-assisted phenogenomic screening in human iPSC disease models, enhanced by AI-driven predictions for efficient target identification and validation. Furthermore, in vivo confirmation validates these findings.
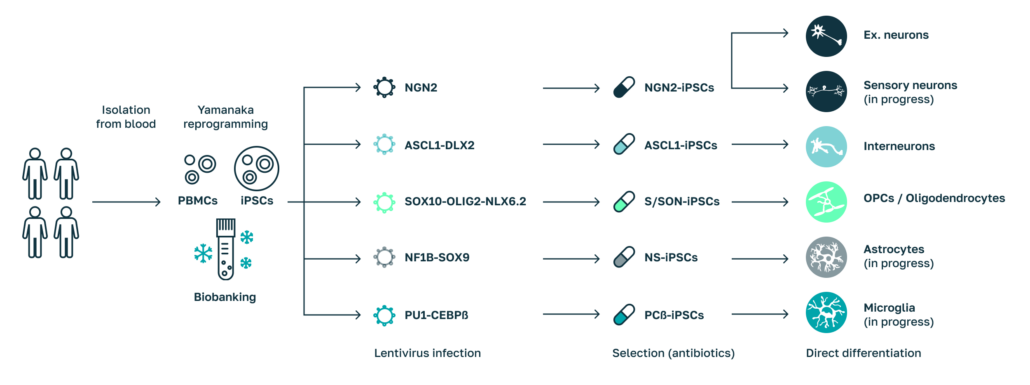
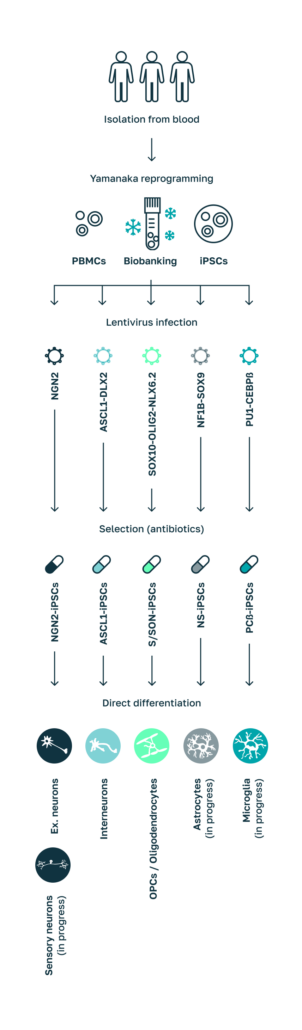
Differentiation of Cell types for Human Disease Models.
Differentiation of Cell types for Human Disease Models.
Innovation is part of our DNA!
Ready to take your research to the next level? Contact us today to learn more about our services, pricing, and how we can help you achieve your scientific goals.
Ready to take your research to the next level? Contact us today to learn more about our services, pricing, and how we can help you achieve your scientific goals.